With contributions from: Matthew Howard, and Cassandra Zuluaga
“Representation Matters” and “Diversity, Equity, and Inclusion (DEI)” are commonly used phrases across healthcare today, but exactly how industry stakeholders should respond to increased calls for diversity and equity is still unclear to many industry leaders. While it may appear obvious that the implications of DEI are especially critical within drug development and clinical trials, many organizations have struggled to mobilize their organizations to take tangible steps to reach their related DEI aims. Ultimately, to recommend a treatment to patients, physicians must have reassurance that the drug has been tested on a representative set of people with successful outcomes.
In this insight, we will cover how to integrate DEI within key steps of the clinical trials process to facilitate better outcomes for both patients and pharmaceutical companies alike. Although meaningful progress will require significant investment from pharmaceutical companies in the areas discussed, there is equally significant upside in prioritizing DEI initiatives.
Specifically, pharmaceutical companies who prioritize DEI in product development can expect:
The business driver of investing in DEI in clinical research is clear: Pharmaceutical companies who choose not to build DEI into their business strategy are missing out on an opportunity to expand the reach of life-changing therapies while isolating large populations of potential consumers.
Good science, and good business, are inherently inclusive.
Setting the stage: A legacy of inequity in healthcare
Disparities in healthcare exist across the entire global healthcare landscape. Examples are readily available and apparent, as patient populations who don’t fit the historically prioritized, ‘default’ profile of “white, cisgender, heterosexual, upper middle-class male” often experience challenges engaging with healthcare organizations and companies worldwide. Two important factors impact how marginalized communities interact with the healthcare industry: distrust of the industry itself and accessibility/awareness of healthcare options.
The historical legacy of healthcare is fraught with injustices such as medical experimentation on enslaved people, the Tuskegee Study, and the categorization of homosexuality as a mental illness diagnosis in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM) prior to 1973. Unfortunately, current events have only escalated the existing distrust within marginalized communities. For example, in February 2015, a 6-day-old infant was turned away by a pediatrician in Michigan because of her same sex parents. Additionally, 28% of trans and gender non-conforming people have postponed medical care when sick or injured due to discrimination and disrespect. It is clear from these recent examples that the healthcare system a whole has a long way to go in terms of providing accessible and equal care to LGBTQ+ populations. As another example, in the UK, it has been reported that Black people have been more fearful of receiving the COVID-19 vaccination due to historic mistrust in pharmaceutical companies. “[T]hat fear meant many people waited, watching to see other people be vaccinated before they felt confident it was safe – all the while risking being exposed to illness.”
That said, historical distrust is not always why individuals from marginalized communities avoid engaging in healthcare. Accessibility and awareness also play an integral part in who engages in healthcare, especially when it comes to participation in clinical trials. Factors such as transportation to a doctor’s office, ability to take time off for visits, and even money for parking can all impact a person’s ability to participate in a clinical trial. Additionally, awareness of clinical trials is not the same across all populations. Some physicians and researchers argue that pharma is actively leaving out marginalized community members from clinical trial outreach efforts as opposed to those community members simply abstaining from participation: “Generally speaking, there’s a segregated healthcare system,” UNC’s Dr. Fisher says in an Atlantic article on clinical trial inclusion. “It’s really not a question of who’s willing to participate. It’s who’s being asked.”
Pharmaceutical companies must take accountability to rebuild trust, amend practices that have alienated marginalized communities, and commit to improving access for these communities after years of mistreatment and neglect.
Present-day shortfalls in drug discovery innovation
While the primary focus of this insight is on clinical trials – we acknowledge the importance of considering DEI in earlier stages of the drug lifecycle.
Technological advances in the 21st century significantly transformed the drug discovery and development process. Currently, 33 pharmaceutical companies are using artificial intelligence to drive drug development, and while technology advances have the power to accelerate drug development, improve therapeutics, and ultimately save lives, they can also widen the equity gap in how people of different genomic backgrounds respond to treatment.
For example, today, 91% of genomic materials available to scientists are of European ancestry. If this material is then informing treatment decisions during research and development across the pharmaceutical industry, it could be deduced that there may be significant gaps in the reached conclusions. From the other side, heterogeneity of genetic markers and pharmacokinetics and pharmacodynamics (PK-PD)— which are the complex, biochemical interactions that occur between the body’s natural processes and the chemical composition of a drug— can account for variability in the efficacy of a drug, as well as variability in drug interactions ideal dosage for varying populations as seen in the following examples:
Underrepresented populations may be left to wonder whether specific therapies will be effective for them, and thus, representative clinical trials are more important now than ever before to ensure the effectiveness and safety of drugs for all patients.
Framework for transformation: Integrating DEI throughout clinical trials
Awareness around historical and current-day shortcomings in promoting an inclusive clinical trial culture has grown as similar movements gained momentum in other industries during the mid-to-late 2010s. DEI is rightfully a hot topic in clinical research as the inherent link to effective science is clear. The industry is asking one critical question:
How can we ensure that medicines work for everyone if clinical trial populations are not diverse?
Trials must be representative to ensure safe, effective treatments. However, driving DEI in clinical trials is more than securing a diverse population set. Across the four key stages of a clinical trial (trial design, study start up, study conduct, and study close out), there are various touch points for industry stakeholders to consider DEI.
A DEI perspective should be embedded within, and considered across, all facets of the clinical research — it cannot be a ‘bolt-on’ to a single component of clinical trial operations, or the remit of a single function to improve. The following is a list of topics which need to be accounted for to maximizing the power of DEI across the clinical trial lifecycle:
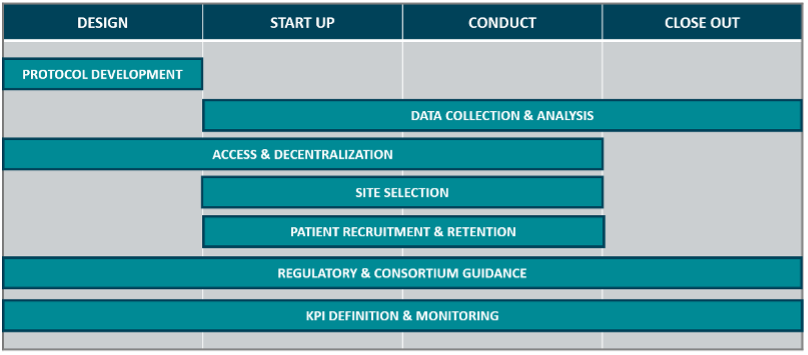
Opportunities for DEI integration
Protocol development
For effective therapies to be developed for people of all backgrounds, research study protocols must be developed to ensure underrepresented populations can participate equally in clinical trials. One way for pharmaceutical companies to address representation limitations is by analyzing historical exclusion criteria. Exclusion criteria are a set of predefined definitions that are used to identify subjects who will not be included or who will have to withdraw from a research study after being included for either ethical reasons (e.g., pregnant women or children in early stage trials) or to eliminate factors that will impact study results (e.g., patients with comorbidities that could impact drug efficacy). While exclusion criteria act as a practical way to ensure drug safety and successful trial results, overly restrictive criteria can limit participation from key patient populations.
Exclusion criteria should be fit for purpose and analyzed to ensure representative trial participation. Consider this example:
It is critical to consider how therapies will impact different patient populations when developing clinical trial protocols. Leveraging traditional demographic markers presents logistical challenges, and more seriously, may further exacerbate inequities in protocol development. Both topics are discussed in more detail below.
Many physicians “still lack consensus on the meaning of race” in clinical research. Race categories are social constructs – they change over time (e.g., the definition of “white” in the United States once excluded eastern European immigrants), are inconsistent across countries, and fail to identify additional genomic markers that impact drug response. For this reason, race considered by itself is an ineffective proxy for representative trials. Instead, data must be analyzed holistically when making inferences regarding the effectiveness of drugs across patient populations. Examples of diversity dimensions that may be leveraged in conjunction with race are shown below:
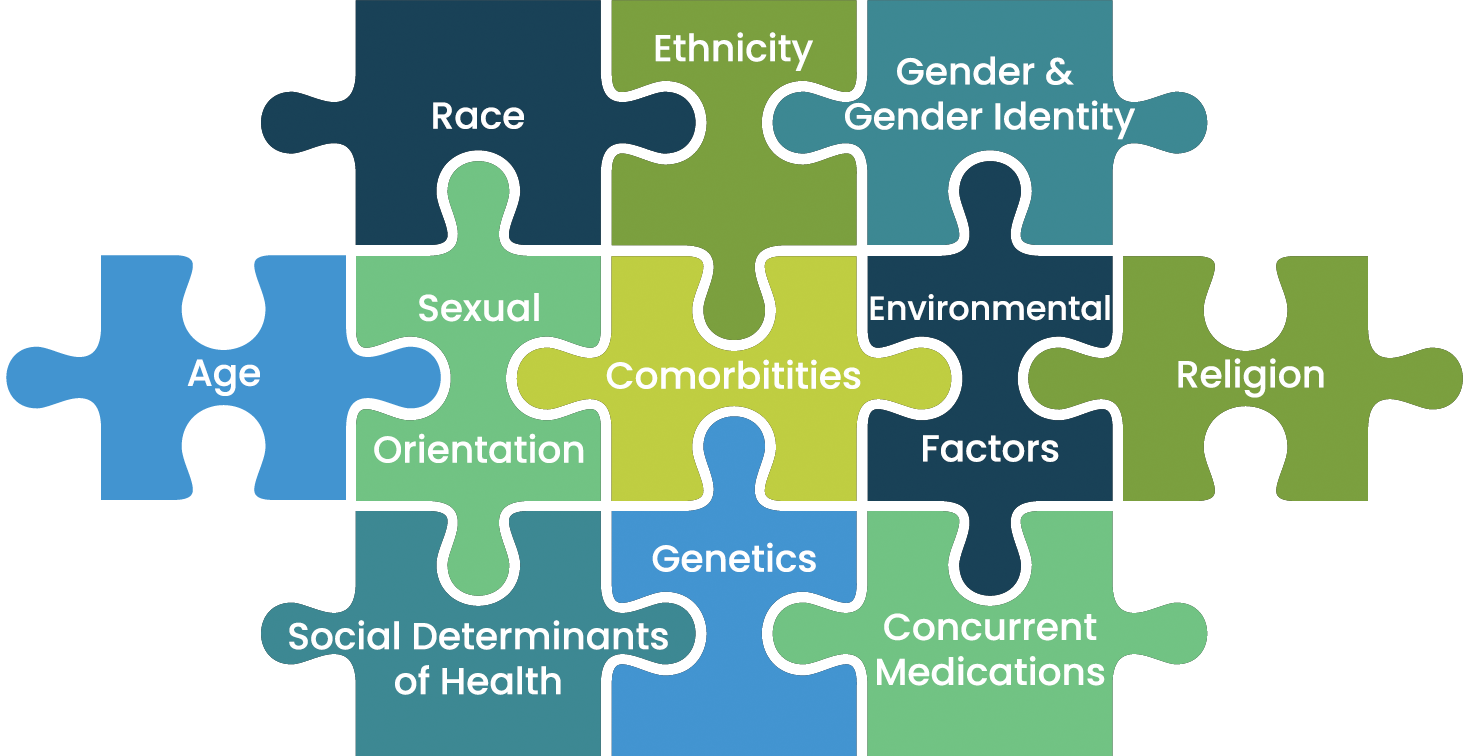
While gaps remain in global, industry-wide standards for demographic classification, we recommend organizations leverage existing standards where possible (e.g., CDISC for U.S., Japan, Europe, and China) and consider demographic variables in context of the specific disease or condition being studied (e.g., gender and sexual orientation for HPV, socioeconomic status for obesity, etc.).
Another serious consideration when leveraging demographic variables for protocol development is the risk of exacerbating the inequities that you are trying to prevent. For example, many race-adjusted algorithms for clinical decisions propagate racial inequities by weighing non-white populations as lower risk for health complications, encouraging providers to direct more attention and resources to white patients than members of racial and ethnic minorities. If using any race-adjusted algorithms to define biomarker thresholds for inclusion/exclusion criteria, collect data during the trial, or conduct downstream analysis, we recommend understanding the historical context behind these adjustments and considering associated equity risks.
Data collection & analysis
There are opportunities for the integration of DEI in statistical analysis from both a technical and cultural lens, discussed below.
From a technical lens:
Protocols must be developed with downstream statistical analysis in mind. They should define the method of outcome analysis for subgroups, leveraging a combination of the defined demographic variables as well as more holistic data.
Traditionally, statistical analysis of subgroups has focused on ruling out inconsistencies: there is an assumption that treatment is equally effective in all subgroups, and the analysis is to determine whether there is evidence to the contrary. More robust analyses are being explored to provide affirmative evidence for differences between subgroups. Another point of integration for DEI in statistical analysis is the consideration of intersectionality — individuals are always a combination of multiple demographic variables (e.g., lesbian, white women may have different outcomes than straight, Black women), and thus, subgroup analysis needs to take multivariate outcomes into account.
From a cultural lens:
Subgroup analysis adds time and complexity to an already intensive research process, and results are often directional at best due to smaller sample sizes. Given a primary driver of clinical trials is to reach endpoints in the shortest amount of time possible, researchers may feel incentivized to deprioritize dedicated analysis to diverse populations.
Therefore, R&D sponsorship around subgroup analysis and reporting is important, both in protocol development and data analysis. Leaders should consider expanding clinical trial success metrics beyond speed alone to the strength of evidence generated across diverse populations. While speed to market is important from a business and competitive lens, being able to generate targeted analyses and associated messaging for specific demographics will provide you with a competitive edge, while also doing right by your patients.
Even if results are not perfect or it takes time to figure out, we cannot progress towards health equity for diverse patient populations by ignoring the dimensions that define this diversity in the analysis of trial outcomes.
Access & decentralization
Although clinical trials can provide access to life-saving therapies, participation can be time-consuming and cost prohibitive. Economic and geographic barriers may impact the ability for representative populations to participate in clinical trials. Nearly 70 percent of potential trial participants in the United States live more than two hours away from the nearest study center.
As digitization in healthcare takes hold following COVID-19 and emerging technologies improve, industry stakeholders continue to assess decentralized clinical trial tools to expand patient diversity and access. The UK government has outlined “patient-centered research – [making] access to and participation in research as easy as possible for everyone across the UK, including rural, diverse, and under-served populations” as a key theme underpinning their vision for the future of clinical research delivery.
Today, studies are designed to decentralize care using the following tools:
Although trials are unlikely to be entirely decentralized with innovative, specialized treatment requiring hands on care, leaders at pharma and contract-research organizations should explore decentralized trials as one method to reach a more representative cohort of participants.
However, while this shift is expected to facilitate more diverse patient populations, it should also be noted that decentralized clinical trials could expand the equity gap in clinical research for several reasons:
During study design and throughout study conduct, pharmaceutical companies must continually assess whether decentralization will have the intended effect of expanding access to underrepresented patient populations.
Site selection
Historically, pharma sponsors have reduced clinical trial cycle time and increased their return on investment by disproportionately allocating study resources and funds to large academic medical institutions and associated PIs. While these institutions and PIs tout improved quality of care, state-of-the-art technology, and access to resources unavailable to smaller community hospitals, diversifying clinical trial participation requires bringing research to the hospitals where patients are receiving care. One of the most critical barriers to enhancing DEI in clinical trials is the lack of sites and PIs in underrepresented communities such as rural and less affluent urban areas.
To bridge the gap between large academic medical institutions and smaller community hospitals, investments in technology and infrastructure must be made. Increased government and public funding can provide community hospitals access to new technologies and tools that make the site attractive to accomplished PIs, sponsors, and site networks performing research.
Although funding technology advancements at community hospitals is foundational to increased representation in clinical research, we cannot bridge the equity gap in clinical research unless all industry participants are invested in the shifting clinical research paradigm. Incentivization is a critical consideration in addressing hesitant industry participants who may benefit under the current paradigm. While ethical implications of incentivization must be considered, including whether the scientific and humanitarian value of more diverse trials should be enough to facilitate the expansion of sites outside of large academic institutions, monetary incentives such as study grants and technology gifts can accelerate adoption of hospitals in areas with underrepresented communities amongst PIs, sponsors, and site networks during site selection.
Ultimately, expanding traditional site networks has the potential to increase access to underrepresented patient populations to ensure representative participation during clinical trials.
Patient recruitment and retention
While decentralization and expanded site selection can introduce new communities to clinical trials, intentionally recruiting diverse patient populations to studies is foundational to bridging the equity gap in clinical research. Increased enrollment and retention of patients from diverse populations can solve for disparate health outcomes across racial and ethnic patient populations.
Rapid expansion of the use of digital tools (e.g., EHR/EMR platforms) in clinical care have transformed the ability of healthcare professionals to recommend clinical interventions. While recruitment strategies previously focused on cumbersome referrals through large health systems, healthcare professionals can now refer qualifying individuals to trials using data routinely collected during clinical care visits to assess eligibility.
When a UK clinical trial related to a COVID-19 recovery intervention ran into recruitment challenges, the NHS provided a daily flow of COVID-19 test data to the trial team to identify potential trial participants. Although barriers to optimal use of digital tools persist, including limitations of research-focused EHR modules and the ability to contact patients cared for by other providers, efforts like NHS’s DigiTrials approach can expand awareness of trials to underrepresented communities.
Outside of improved qualitative measures afforded via today’s digitally driven environment, additional communications and community outreach programs are critical to driving representative populations to clinical trials. Considerations include the following:
There are many tactics to consider when engaging community stakeholders and promoting clinical trial participation, but community awareness should not stop during clinical trial recruitment. Engagement should be personalized and patient-centric across the patient journey to promote a trusting environment for underrepresented patient communities.
Regulatory & consortium guidance
To assist with the integration of DEI considerations within the end-to-end clinical process, we recommend keeping up to date on relevant guidance from Regulatory bodies and industry consortiums. Below is a list of recent policy and guidance updates:
KPI definition & monitoring
KPI definition and monitoring, particularly for operational performance, is already a standard component of clinical trial execution. We recommend applying a similar rigor and approach to embed DEI-focused KPIs, just as you would for any other category of metrics.
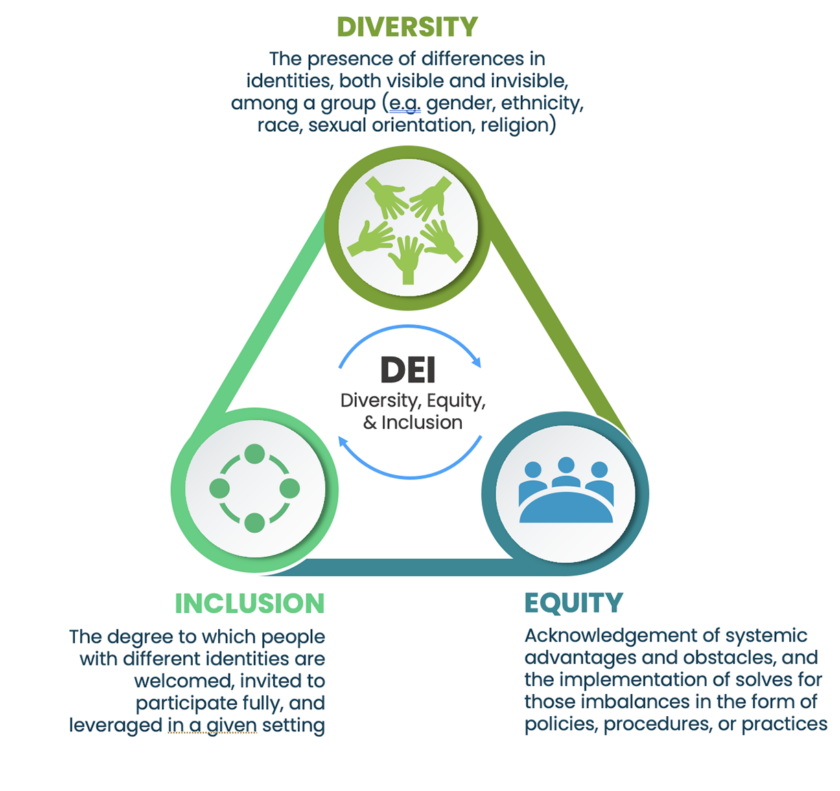
While metrics will be specific to each organization’s clinical trial design and protocol, we generally recommend tying metrics to diversity, equity, or inclusion separately. See below for a reminder of the distinct purposes of DEI (also noted in the introduction):
See below for sample DEI metrics tied to Patient Recruitment and Retention to illustrate why the distinction is important:
In this example, diversity within the clinical trial patient population is important. However, without equitable opportunities for enrollment and access, the trial would not achieve the diversity KPI. And without inclusion, organizations will lose participants from diverse backgrounds before study completion.
Tying DEI metrics to specific initiatives is another critical principle for KPI measurement. However, initiatives intended to bolster DEI can sometimes have the unintentional effect of exacerbating inequities. We shared two examples of this in previous sections: 1) how decentralization of clinical trials could expand reach to diverse patient populations or leave behind communities with limited access to technology and 2) how leveraging demographic markers to identify patients may result in propagation of racial bias in existing clinical algorithms. Healthcare organizations should not assume that initiatives have the intended results; therefore, assessing DEI strategies through the monitoring and review of KPIs is critical. Transformation of DEI in clinical research and greater representation will require iterative solutions and continual, incremental progress.
Although shortcomings around DEI have long impacted clinical trials, there are opportunities to refocus and amend processes to be more inclusive and equitable. Incremental and intentional progress across independent activities throughout the clinical trial lifecycle will improve disparities in healthcare and deliver better outcomes for both patients and pharmaceutical companies. Industry transformation is also dependent on large and small companies embedding a DEI mindset within their organization and assessing DEI strategy holistically.
Vynamic’s Diversity, Equity, & Inclusion service helps clients define and support DEI within their organization to enhance business strategy, organizational dynamics, and team member experience. To explore how Vynamic can help your organization achieve its DEI goals, please contact DEI Service Leads, Shannon Connolly and Stefanie Christmas, and Head of Life Sciences Sector, Karen Baldry.
For additional information on DEI in Clinical Trials, check out the Trending Health podcast episode “DEI & Clinical Trials – Driving Meaningful Change” and case study “Prioritizing Diversity, Equity, & Inclusion Across Research & Development.”
View all references and end notes here on pg.10.
*Clinical Trial Diversity is Key to Future Innovation | Abbott U.S. (n.d.). Retrieved May 23, 2022, from https://www.abbott.com/diversity-in-clinical-trials.html
*Drescher, J. (2015). Out of DSM: depathologizing homosexuality. Behavioral Sciences (Basel, Switzerland), 5(4), 565–575. https://doi.org/10.3390/bs5040565
*“You Don’t Want Second Best”: Anti-LGBT Discrimination in US Health Care | HRW. (n.d.). Retrieved May 23, 2022, from https://www.hrw.org/report/2018/07/23/you-dont-want-second-best/anti-lgbt-discrimination-us-health-care
*Grant, J. M., Mottet, L. A., Tanis, J., Herman, J. L., Harrison, J., & Keisling, M. (2010). National Transgender Discrimination Survey Report on Health and Health Care. National Center for Transgender Equality, National Gay and Lesbian Task Force. https://cancer-network.org/wp-content/uploads/2017/02/National_Transgender_Discrimination_Survey_Report_on_health_and_health_care.pdf
*Schraer, R. (2021, May 6). Covid: Black leaders fear racist past feeds mistrust in vaccine. BBC News. https://www.bbc.com/news/health-56813982
*Jacewicz, N. (2016, July 16). Why Are Health Studies So White? The Atlantic. https://www.theatlantic.com/health/archive/2016/06/why-are-health-studies-so-white/487046/
*St. Fleur/STAT News, N. (2021, April 28). Virtual event: Increasing diversity in clinical trials. https://www.statnews.com/2021/04/28/virtual-event-increasing-diversity-in-clinical-trials/
*Hartford, C., & Pulliza, C. (2021, April 30). FPM Conversation Blog – Enabling a Greater Diversity of People in Clinical Trials. Faculty of Pharmaceutical Medicine. https://www.fpm.org.uk/blog/fpm-conversation-blog-enabling-a-greater-diversity-of-people-in-clinical-trials/
*American Cancer Society. (2018, April 27). What Is Breast Cancer in Men? https://www.cancer.org/cancer/breast-cancer-in-men/about/what-is-breast-cancer-in-men.html
*Vyas, D. A., Eisenstein, L. G., & Jones, D. S. (2020). Hidden in Plain Sight – Reconsidering the Use of Race Correction in Clinical Algorithms. The New England Journal of Medicine, 383(9), 874–882. https://doi.org/10.1056/NEJMms2004740
*Starkey, B. S. (2017, February 10). White immigrants weren’t always considered white – and acceptable. Andscape. https://andscape.com/features/white-immigrants-werent-always-considered-white-and-acceptable/
*Bierer, B., White, S., Meloney, L., Ahmed, H., Strauss, D., & Clark, L. (2020). Achieving Diversity, Inclusion, and Equity in Clinical Research. MRCT Center of Brigham and Women’s Hospital and Harvard.
*Sanofi. (2017, March 2). Sanofi Launches Digital Clinical Trials to Improve Recruitment and Reduce Trial Times. https://www.sanofi.com/en/media-room/articles/2017/sanofi-launches-digital-clinical-trials-to-improve-recruitment-and-reduce-trial-times
*UK Government. (2020, March 23). Executive Summary: The Future of UK Clinical Research Delivery. Gov.UK. https://www.gov.uk/government/publications/the-future-of-uk-clinical-research-delivery/executive-summary
*Agrawal, G., Moss, R., Raschke, R., Wurzer, S., & Xue, J. (2021). No place like home? Stepping up the decentralization of clinical trials. McKinsey & Company. https://www.mckinsey.com/industries/life-sciences/our-insights/no-place-like-home-stepping-up-the-decentralization-of-clinical-trials
*NHS Digital. (2022). NHS DigiTrials: Already saving lives. National Health Systems. https://digital.nhs.uk/features/nhs-digitrials-already-saving-lives
*O’Brien, E.C., Raman, S.R., Ellis, A. et al. The use of electronic health records for recruitment in clinical trials: a mixed methods analysis of the Harmony Outcomes Electronic Health Record Ancillary Study. Trials 22, 465 (2021). https://doi.org/10.1186/s13063-021-05397-0
*Borough Profile 2018: Tower Hamlets. (2018). Tower Hamlets Corporate Research Unit. https://www.towerhamlets.gov.uk/Documents/Borough_statistics/Research-briefings/Population_2_BP2018.pdf.
*Jensen, B. (2020). Boosting Diversity in COVID-19 Clinical Trials. Johns Hopkins University. https://hub.jhu.edu/2020/11/30/diversity-covid-19-vaccine-trials/
*Office of Minority Health. (2016). Collection of Race and Ethnicity Data in Clinical Trials – Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration.
*Center for Biologics Evaluation and Research, & Center for Drug Evaluation and Research. (2020). Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry (FDA-2019-D-1264). Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial
*Oncology Center of Excellence, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health, & Office of Minority Health and Health Equity. (2022). Diversity Plans to Improve Enrollment of Participants From Underrepresented Racial and Ethnic Populations in Clinical Trials; Draft Guidance for Industry; Availability (FDA-2021-D-0789). Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations
*Pharmaceutical Research and Manufacturers of America. (2020). Principles on Conduct of Clinical Trials, Communication of Clinical Trial Results. Pharmaceutical Research and Manufacturers of America. https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/P-R/PhRMAPrinciples-of-Clinical-Trials-FINAL.pdf
*Younossi, A., Sanhai, W., Shah, S., & Chang, C. (2021). Enhancing clinical trial diversity. Deloitte. https://www2.deloitte.com/us/en/insights/industry/life-sciences/lack-of-diversity-clinical-trials.html
*Transcelerate Biopharma, Inc. (2022). Patient Experience Solutions. https://www.transceleratebiopharmainc.com/assets/patientexperience/
*Transcelerate Biopharma, Inc. (2022). Diversity of Participants in Clinical Trials Solutions. https://www.transceleratebiopharmainc.com/initiatives/diversity-of-participants/
*Momoh, O., Burriss, S., Harry, A., & Warner, K. (n.d.). Inclusion & Diversity in Clinical Trials. EUPATI. Retrieved May 23, 2022, from https://toolbox.eupati.eu/resources/inclusion-diversity-clinical-trials/
*Grine, L., Janssens, R., van Overbeeke, E., Derijcke, D., Silva, M., Delys, B., Dusart, I., Aertsen, V., Mertens de Wilmars, M., Robaczewska, J., & Stevens, H. (2020). Improving patient involvement in the lifecycle of medicines: insights from the EUPATI BE survey. Frontiers in Medicine, 7, 36. https://doi.org/10.3389/fmed.2020.00036
*European Medicines Agency. (2020). EMR Regulatory Science to 2025. European Medicines Agency. http://EMA Regulatory Science to 2025
Vynamic, an Inizio Advisory company, is a leading management consulting partner to global health organizations across Life Sciences, Health Services, and Health Technology. Founded and headquartered in Philadelphia, Vynamic has offices in Boston, Durham NC, New York, and London. Our purpose is simple: We believe there is a better way. We are passionate about shaping the future of health, and for more than 20 years we’ve helped clients transform by connecting strategy to action.
Through a structured, yet flexible delivery model, our accomplished leaders work as an extension of client teams, enabling growth, performance, and culture. Vynamic has been recognized by organizations like Great Place to Work and Business Culture Awards for being leaders and innovators in consulting, company culture, and health. Visit Vynamic.com to discover how we can help transform your
organization or your career.
 Tamaron Bertelli
Tamaron Bertelli
This structured approach guides Med Affairs leaders in executing micro-transformations to drive meaningful impact for your organization and patients.
Read more Jess Werle
Jess Werle
Embrace MedTech's future by blending field and digital strategies to boost sales effectiveness, unleash the power of data, and stand out in the market
Read more Jack Young
Jack Young
Explore how leading with authenticity not only ensures success in the launch of life-saving medicines, but generates positive business results too.
Read more