Catching FHIR®: Secondary use of EHR data for clinical research
Learn how Vynamic continues to support a cutting-edge effort to improve data interoperability for clinical research by leveraging clinical data from investigator site EHRs.
The challenge
The healthcare industry continues to experience electronic health data interoperability challenges between patients, research investigator sites, sponsors, and external vendors. Data is often siloed, and many standards are used across the industry which can negatively impact data quality, timeliness, usability, and overall clinical trial experience for both clinicians and patients. Vynamic was approached by a global pharmaceutical leader to provide strategic program leadership for their cutting-edge eSource program, which aims to define the future of secondary use of EHR data for clinical research.
The approach
Because government regulations require increased adoption and use of electronic data standards, data interoperability in clinical care has been a major industry focus in recent years. Data interoperability in clinical research has lagged behind other areas due to the complex ecosystem in which Pharma companies operate. As the industry races to define the future of data interoperability in clinical research, Vynamic’s approach as a partner needed to account for fast-moving, iterative product and process development, while creating an actionable strategy to scale the eSource Program in a myriad of ways. The team focused on five key areas:
Technology transformation
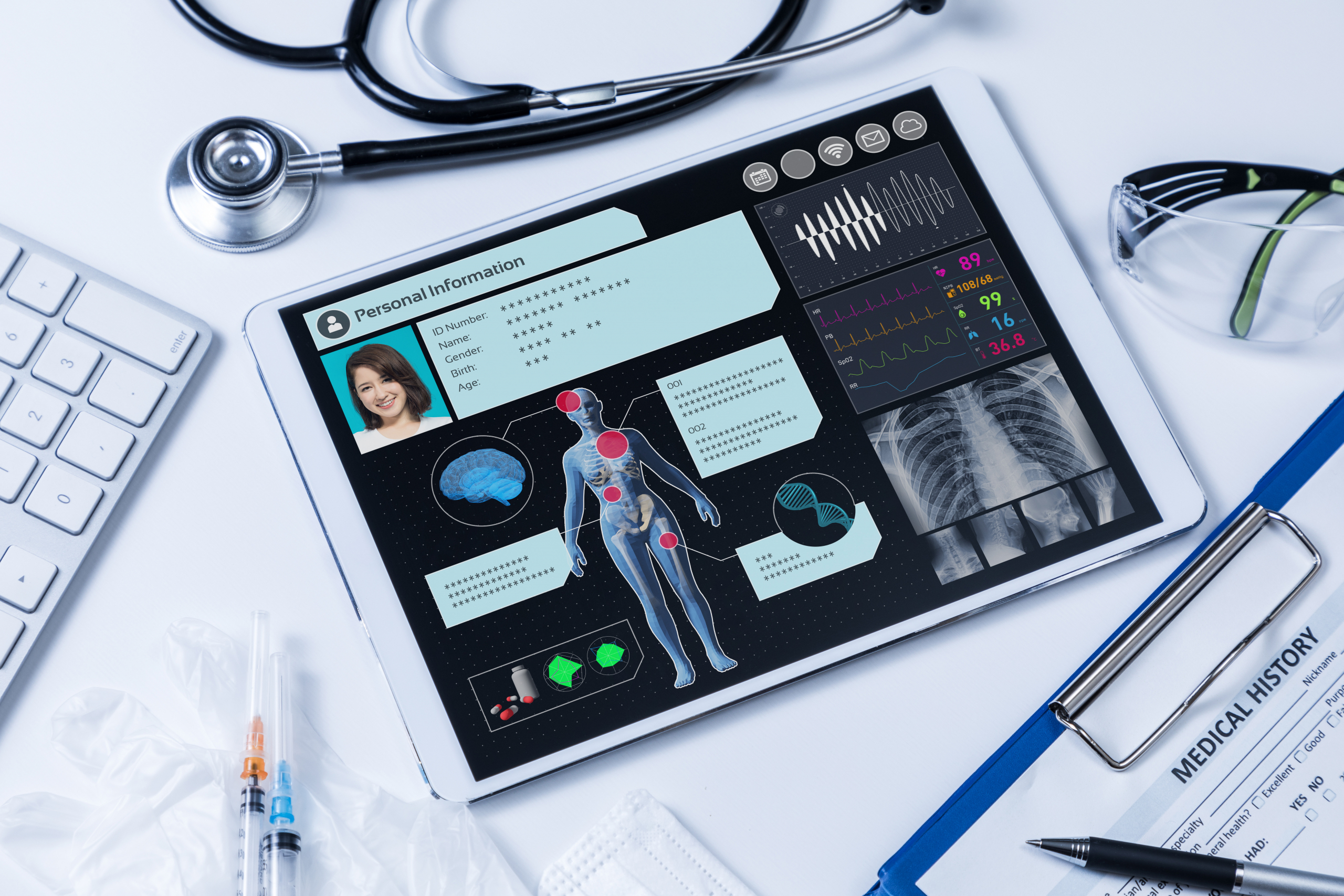
Automated the clinical trial data exchange process and removed the need for manual intervention and dual documentation for select data domains by leveraging a made-for-purpose mapping template, based upon HL7® FHIR® standards, to normalize and aggregate data from disparate sources and push to our client’s EDC system.
Stakeholder management
Developed and led an evolving support team operating model to scale from initial solution to business-as-usual application, and aligned internal business and technology stakeholders with external technology vendors through project execution and managed project relationships with key clinical study site partners, including several of the leading academic health systems in the US.
Business process enablement
Supported the development and redesign of end-to-end business processes defining data capture operations through the clinical research lifecycle and supported impact analysis, training, and upskilling efforts as domain SMEs.
Strategic program management
Employed a data-driven approach to assess new potential strategic partners to scale first-of-its-kind solution to additional clinical research sites and studies across therapeutic areas and geographies.
Industry engagement
Supported client engagement in multiple industry consortia, including the HL7® Vulcan® Accelerator for research to connect cross-sector participants and progress interoperability solutions, and developed material to be shared at various industry conferences.
The results
Vynamic’s efforts have facilitated the design of an industry-first data interoperability solution that automates the use of EHR data for clinical research using HL7® FHIR®. This cutting-edge effort improves data quality for clinical research, reduces the burden of data capture for investigator sites and research study teams, and will ultimately enhance the overall clinical trial experience for patients and physicians alike. Vynamic’s efforts have helped move the entire healthcare industry forward as eSource streamlines data interoperability in clinical research.
Learn more about eSource on Vynamic’s Trending Health podcast here.
Learn more about the HL7® Vulcan® Accelerator, dedicated to connecting clinical research and healthcare, by watching this video. Learn more about joining here.
About Vynamic
Vynamic, an Inizio Advisory company, is a leading management consulting partner to global health organizations across Life Sciences, Health Services, and Health Technology. Founded and headquartered in Philadelphia, Vynamic has offices in Boston, Durham NC, New York, and London. Our purpose is simple: We believe there is a better way. We are passionate about shaping the future of health, and for more than 20 years we’ve helped clients transform by connecting strategy to action.
Through a structured, yet flexible delivery model, our accomplished leaders work as an extension of client teams, enabling growth, performance, and culture. Vynamic has been recognized by organizations like Great Place to Work and Business Culture Awards for being leaders and innovators in consulting, company culture, and health. Visit Vynamic.com to discover how we can help transform your
organization or your career.
Want to learn more? Get in touch!
Other insights.
Jump to a slide with the slide dots.
 Sean Martin
Sean Martin
Strategic Transformation for a Top 10 Life Sciences Company
A top 10 life sciences firm drove $300M impact via enterprise-wide transformation, PMO scaling & strategy to tackle the patent cliff.
Read more Sean Martin
Sean Martin
Transforming the Commercial & Medical Organization for a Global Biopharma Leader
Vynamic helped a global biopharma firm rapidly transform its Commercial & Medical model, achieving 97% of key changes and enabling strategic growth.
Read moreHCP and Consumer Omnichannel Transformation
Vynamic enabled omnichannel marketing, AI-driven sales, and governance, boosting HCP engagement, consumer reach, and sales impact.
Read more