Transition framework development
The challenge
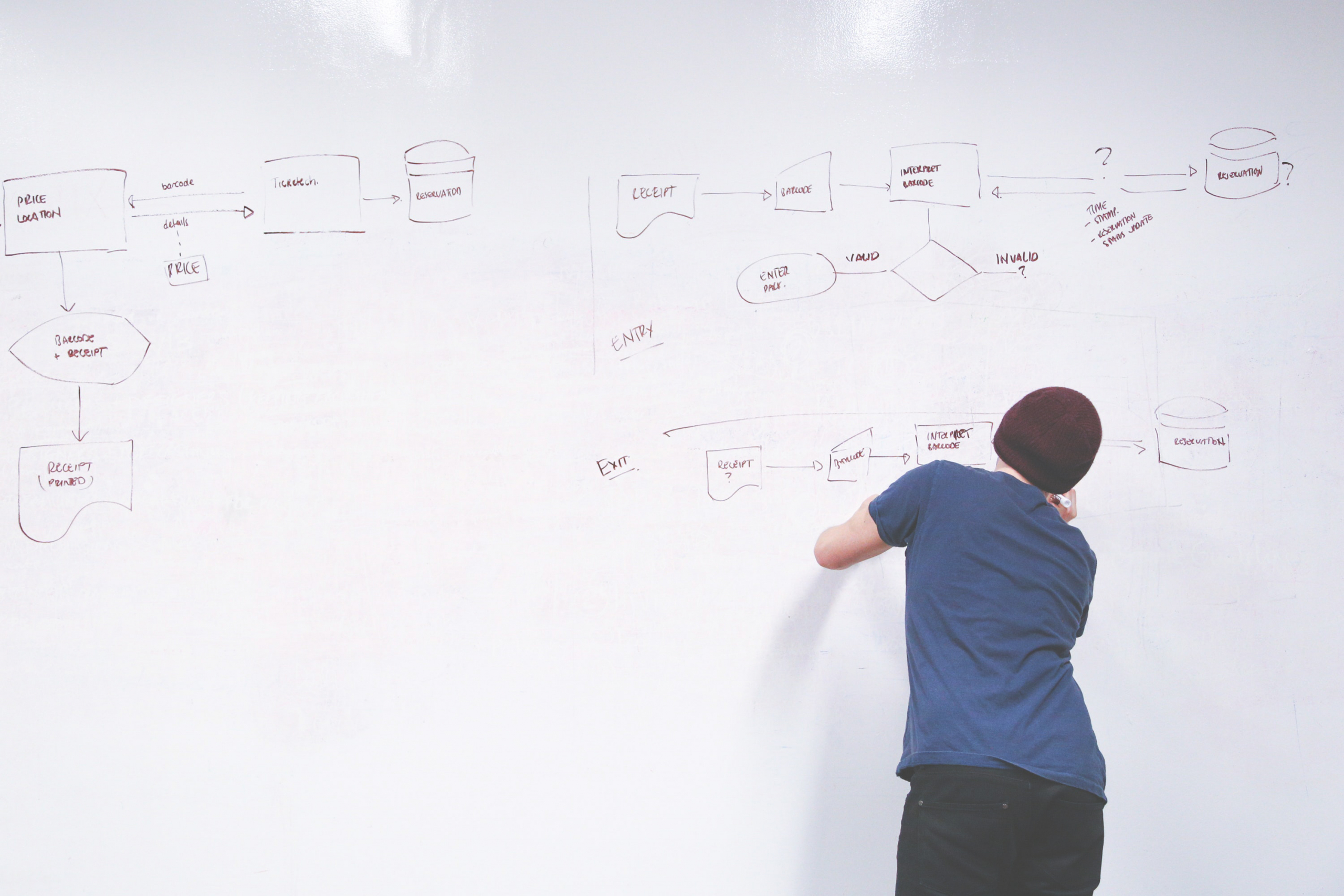
Medical Affairs (MA) at a large global pharmaceutical company required a structured framework to transition products into the late-lifecycle products portfolio. The organization needed a consistent and optimized approach through the development of an adaptable, modular framework to account for product diversity and situation.
Our approach
Vynamic mapped the internal organization across functions and roles into a RACI with senior management buy-in. Then a draft Transition Framework was developed, followed by validation at several organizational levels and a beta test.
The results
- An industry leading Transition Framework ready for trial with outstanding organizational feedback.
- A Utilization Guide and Tools Suite was also developed, communicated, and rolled-out with KPIs.
About Vynamic
Vynamic, an Inizio Advisory company, is a leading management consulting partner to global health organizations across Life Sciences, Health Services, and Health Technology. Founded and headquartered in Philadelphia, Vynamic has offices in Boston, Durham NC, New York, and London. Our purpose is simple: We believe there is a better way. We are passionate about shaping the future of health, and for more than 20 years we’ve helped clients transform by connecting strategy to action.
Through a structured, yet flexible delivery model, our accomplished leaders work as an extension of client teams, enabling growth, performance, and culture. Vynamic has been recognized by organizations like Great Place to Work and Business Culture Awards for being leaders and innovators in consulting, company culture, and health. Visit Vynamic.com to discover how we can help transform your
organization or your career.
Want to learn more? Get in touch!
Other insights.
Jump to a slide with the slide dots.
Strategic Transformation for a Top 10 Life Sciences Company
A top 10 life sciences firm drove $300M impact via enterprise-wide transformation, PMO scaling & strategy to tackle the patent cliff.
Read moreTransforming the Commercial & Medical Organization for a Global Biopharma Leader
Vynamic helped a global biopharma firm rapidly transform its Commercial & Medical model, achieving 97% of key changes and enabling strategic growth.
Read moreHCP and Consumer Omnichannel Transformation
Vynamic enabled omnichannel marketing, AI-driven sales, and governance, boosting HCP engagement, consumer reach, and sales impact.
Read more